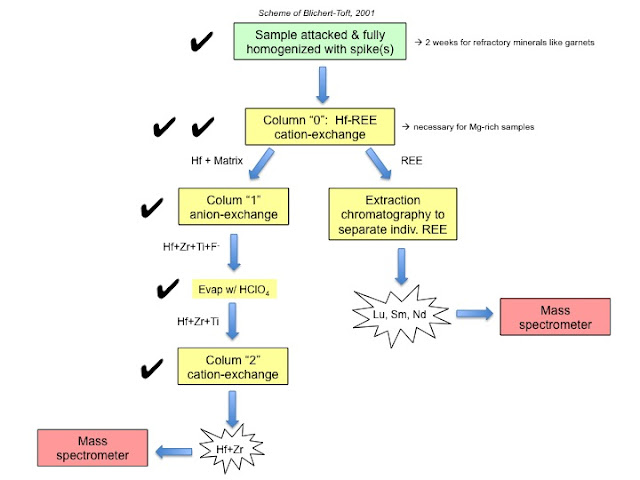
So now, my Hf fraction is ready to go on the mass spectrometer! The only other chemistry left to do is the separation of the REE.
To pick up where we left off from Column "1". After Column "1", we're left with our sample stripped of everything else except for Hf, Zr, and Ti. In Column "1", a dilute solution of HF and HCl was used so that Hf, Zr, and Ti "stuck" onto the resin (via complexation with the F- in HF. Amazingly, the F- is so strong that this works even in such a dilute solution!). While those elements were sticking on to the resin, all the other elements were stripped away. Finally, to remove the stuck Hf, Zr, and Ti, we eluted with a stronger concentration of HCl (6 M).
So, at this point, all we have left to do is remove Ti, which is what Column "2" is for. Column "2" is by far the most spectacular looking (IF everything in the previous 2 columns went perfectly!). The reason is because the very first step of Column "2" is the addition of a small amount of H2O2 to the sample. H2O2 complexes with the high field strength elements. All the complexes are colorless, with the exception of the Ti complex - this one is bright orange-red. We definitely had a highly nervous moment when we added the H2O2 to my samples (everyone in the lab watched) - because if nothing turned red, that means the Hf and Ti got lost somehow earlier. Thankfully, all the samples did change color, meaning all the previous columns ran fine. Below, here's the sample + H2O2 loaded onto the column. You can see the bright red bands that represent Ti:
Below, here are two subsequent photos of the column being run. So cool how you can watch the Ti (well, watch it very slowly... the narrowness of this column means it takes ~1 hour or more for each elution step):
So, that was it. All hafnium chemistry done. The final thing I did was then to evaporate the Hf separates. So there's about 6 ml of solution at the final step, and after it was completely dried down, all that was left was literally a SPECK in the bottom of the beaker. That speck is all the Hf and Zr contained in the sample (well, not exactly 100%, but we're hoping at least 90%). That was definitely a cool moment - after three columns, all we have left is a few black specks.
One thing I have learned about column chemistry is that it can be tedious and take a long time. Particularly when you re-use columns (which is a good thing, because resin is very expensive). Re-using columns means they must be cleaned exceptionally well, which is often a whole-day affair, if not more. I'm amazed by the amount of acid we have gone through in running columns and cleaning them. With three people running Hf chemistry in the lab, we've probably gone through a liter of HF just in cleaning columns, and more for HCl. And that's just in 3 weeks. At one point, we ran out of acid and had to wait a few days for it to be distilled. Then after distillation, it has to be titrated - which in itself can take an entire afternoon. Then there is the continual washing and cleaning of beakers so that everyone has enough stuff to use. It's definitely a lot of work to run and maintain an isotope geochemistry lab!
That was a bit of a stressful week, but the nice thing is now I have a couple days to see as much more of Lyon as possible. Unfortunately the weather took a nasty turn here: temperatures around 35 F, rainy, and with a biting wind. Of course, that has to happen the last few days I am here, while all of October was wonderfully pleasant and anomalously warm. I wonder if this sudden change to freezing weather has something to do with Hurricane Sandy moving along the Eastern US Seaboard. The North Atlantic was unusually warm this year, with sea surface temperatures over 2 degrees above average. These anomalously warm waters are probably what's feeding the hurricane and allowing it to intensify. Western intensification at its most intense? While the hurricane is sucking up all that warmth, the eastern side of the ocean basin (Europe) isn't getting any more of that warmth, and instead is now freezing (intensification of what would normally happen for cold eastern boundary currents). Anyway, as I write this, I can hear the wind roaring outside and I even had to turn the heat on.
So the bad weather is keeping me indoors for now, and giving me some time to work on some job applications. My brain is kind of dead after this crazy week, so I just decided to work on "easier" stuff like applications, and to relax a bit. I feel like this whole month has been intense. With the nature of my samples being so rare and precious (in the sense that it took 5 months to glean enough sample), every step in the lab had to be orchestrated perfectly. And some parts were pretty dicey. A few samples behaved really oddly, like being strangely viscous and forming a gel at the bottom of the bomb. We then had to stick those back into the oven for several more days until a homogenous solution was obtained. One sample even got stuck in the resin while we were running one of the columns, and lagged behind all the other samples (which meant waiting till late in the evening for it to go through, and hoping it would!). So, I will be amazed if at the end, we actually get a meaningful number out of all this! Even if we don't, I still walked away with an invaluable experience.
While being cooped up inside last night, I decided to finally do some drawing. I used to draw a lot, and took several art classes in college, and have always drawn for fun. I find it a stress reliever - to be able to concentrate on something that just requires hand-eye-brain coordination and nothing intellectual. But I literally have not had time to draw anything serious in months, probably years. Thankfully I still carry around these thin colored pens with me, just in the random event I feel like drawing something. You can get these at any art supply store or even Staples and Officemax - they're Triplus fineliners, made by Staedtler. Pretty inexpensive, and highly portable. I drew two birds I've seen here in France, the blue tit and European robin:
And then I decided to do something more complicated, one of my favorite American birds, the American kestrel:







No comments:
Post a Comment